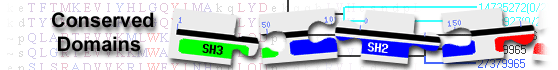hypothetical protein GDO81_012564 [Engystomops pustulosus]
List of domain hits

| Name | Accession | Description | Interval | E-value | |||
| DUS_like_FMN | cd02801 | Dihydrouridine synthase-like (DUS-like) FMN-binding domain. Members of this family catalyze ... |
313-411 | 9.84e-37 | |||
Dihydrouridine synthase-like (DUS-like) FMN-binding domain. Members of this family catalyze the reduction of the 5,6-double bond of a uridine residue on tRNA. Dihydrouridine modification of tRNA is widely observed in prokaryotes and eukaryotes, and also in some archaea. Most dihydrouridines are found in the D loop of t-RNAs. The role of dihydrouridine in tRNA is currently unknown, but may increase conformational flexibility of the tRNA. It is likely that different family members have different substrate specificities, which may overlap. 1VHN, a putative flavin oxidoreductase, has high sequence similarity to DUS. The enzymatic mechanism of 1VHN is not known at the present. : Pssm-ID: 239200 [Multi-domain] Cd Length: 231 Bit Score: 134.16 E-value: 9.84e-37
|
|||||||
| Zn_finger_prot super family | cl44678 | Putative zinc finger protein; This is a family of proteins predominantly found in Iridoviridae, ... |
55-161 | 7.56e-04 | |||
Putative zinc finger protein; This is a family of proteins predominantly found in Iridoviridae, which contains a zinc finger domain. The actual alignment was detected with superfamily member pfam19242: Pssm-ID: 437074 Cd Length: 268 Bit Score: 41.21 E-value: 7.56e-04
|
|||||||
| Name | Accession | Description | Interval | E-value | |||
| DUS_like_FMN | cd02801 | Dihydrouridine synthase-like (DUS-like) FMN-binding domain. Members of this family catalyze ... |
313-411 | 9.84e-37 | |||
Dihydrouridine synthase-like (DUS-like) FMN-binding domain. Members of this family catalyze the reduction of the 5,6-double bond of a uridine residue on tRNA. Dihydrouridine modification of tRNA is widely observed in prokaryotes and eukaryotes, and also in some archaea. Most dihydrouridines are found in the D loop of t-RNAs. The role of dihydrouridine in tRNA is currently unknown, but may increase conformational flexibility of the tRNA. It is likely that different family members have different substrate specificities, which may overlap. 1VHN, a putative flavin oxidoreductase, has high sequence similarity to DUS. The enzymatic mechanism of 1VHN is not known at the present. Pssm-ID: 239200 [Multi-domain] Cd Length: 231 Bit Score: 134.16 E-value: 9.84e-37
|
|||||||
| DusA | COG0042 | tRNA-dihydrouridine synthase [Translation, ribosomal structure and biogenesis]; ... |
308-412 | 1.82e-18 | |||
tRNA-dihydrouridine synthase [Translation, ribosomal structure and biogenesis]; tRNA-dihydrouridine synthase is part of the Pathway/BioSystem: tRNA modification Pssm-ID: 439812 [Multi-domain] Cd Length: 310 Bit Score: 85.53 E-value: 1.82e-18
|
|||||||
| nifR3_yhdG | TIGR00737 | putative TIM-barrel protein, nifR3 family; This model represents one branch of COG0042 ... |
306-411 | 1.85e-15 | |||
putative TIM-barrel protein, nifR3 family; This model represents one branch of COG0042 (Predicted TIM-barrel enzymes, possibly dehydrogenases, nifR3 family). This branch includes NifR3 itself, from Rhodobacter capsulatus. It excludes a broadly distributed but more sparsely populated subfamily that contains sll0926 from Synechocystis PCC6803, HI0634 from Haemophilus influenzae, and BB0225 from Borrelia burgdorferi. It also excludes a shorter and more distant archaeal subfamily.The function of nifR3, a member of this family, is unknown, but it is found in an operon with nitrogen-sensing two component regulators in Rhodobacter capsulatus.Members of this family show a distant relationship to alpha/beta (TIM) barrel enzymes such as dihydroorotate dehydrogenase and glycolate oxidase. [Unknown function, General] Pssm-ID: 129820 Cd Length: 319 Bit Score: 77.02 E-value: 1.85e-15
|
|||||||
| Dus | pfam01207 | Dihydrouridine synthase (Dus); Members of this family catalyze the reduction of the 5,6-double ... |
316-411 | 2.97e-10 | |||
Dihydrouridine synthase (Dus); Members of this family catalyze the reduction of the 5,6-double bond of a uridine residue on tRNA. Dihydrouridine modification of tRNA is widely observed in prokaryotes and eukaryotes, and also in some archae. Most dihydrouridines are found in the D loop of t-RNAs. The role of dihydrouridine in tRNA is currently unknown, but may increase conformational flexibility of the tRNA. It is likely that different family members have different substrate specificities, which may overlap. Dus 1 from Saccharomyces cerevisiae acts on pre-tRNA-Phe, while Dus 2 acts on pre-tRNA-Tyr and pre-tRNA-Leu. Dus 1 is active as a single subunit, requiring NADPH or NADH, and is stimulated by the presence of FAD. Some family members may be targeted to the mitochondria and even have a role in mitochondria. Pssm-ID: 426126 Cd Length: 309 Bit Score: 61.19 E-value: 2.97e-10
|
|||||||
| PRK10415 | PRK10415 | tRNA-dihydrouridine synthase B; Provisional |
310-416 | 6.82e-08 | |||
tRNA-dihydrouridine synthase B; Provisional Pssm-ID: 182440 Cd Length: 321 Bit Score: 53.82 E-value: 6.82e-08
|
|||||||
| Zn_finger_prot | pfam19242 | Putative zinc finger protein; This is a family of proteins predominantly found in Iridoviridae, ... |
55-161 | 7.56e-04 | |||
Putative zinc finger protein; This is a family of proteins predominantly found in Iridoviridae, which contains a zinc finger domain. Pssm-ID: 437074 Cd Length: 268 Bit Score: 41.21 E-value: 7.56e-04
|
|||||||
| Name | Accession | Description | Interval | E-value | |||
| DUS_like_FMN | cd02801 | Dihydrouridine synthase-like (DUS-like) FMN-binding domain. Members of this family catalyze ... |
313-411 | 9.84e-37 | |||
Dihydrouridine synthase-like (DUS-like) FMN-binding domain. Members of this family catalyze the reduction of the 5,6-double bond of a uridine residue on tRNA. Dihydrouridine modification of tRNA is widely observed in prokaryotes and eukaryotes, and also in some archaea. Most dihydrouridines are found in the D loop of t-RNAs. The role of dihydrouridine in tRNA is currently unknown, but may increase conformational flexibility of the tRNA. It is likely that different family members have different substrate specificities, which may overlap. 1VHN, a putative flavin oxidoreductase, has high sequence similarity to DUS. The enzymatic mechanism of 1VHN is not known at the present. Pssm-ID: 239200 [Multi-domain] Cd Length: 231 Bit Score: 134.16 E-value: 9.84e-37
|
|||||||
| DusA | COG0042 | tRNA-dihydrouridine synthase [Translation, ribosomal structure and biogenesis]; ... |
308-412 | 1.82e-18 | |||
tRNA-dihydrouridine synthase [Translation, ribosomal structure and biogenesis]; tRNA-dihydrouridine synthase is part of the Pathway/BioSystem: tRNA modification Pssm-ID: 439812 [Multi-domain] Cd Length: 310 Bit Score: 85.53 E-value: 1.82e-18
|
|||||||
| nifR3_yhdG | TIGR00737 | putative TIM-barrel protein, nifR3 family; This model represents one branch of COG0042 ... |
306-411 | 1.85e-15 | |||
putative TIM-barrel protein, nifR3 family; This model represents one branch of COG0042 (Predicted TIM-barrel enzymes, possibly dehydrogenases, nifR3 family). This branch includes NifR3 itself, from Rhodobacter capsulatus. It excludes a broadly distributed but more sparsely populated subfamily that contains sll0926 from Synechocystis PCC6803, HI0634 from Haemophilus influenzae, and BB0225 from Borrelia burgdorferi. It also excludes a shorter and more distant archaeal subfamily.The function of nifR3, a member of this family, is unknown, but it is found in an operon with nitrogen-sensing two component regulators in Rhodobacter capsulatus.Members of this family show a distant relationship to alpha/beta (TIM) barrel enzymes such as dihydroorotate dehydrogenase and glycolate oxidase. [Unknown function, General] Pssm-ID: 129820 Cd Length: 319 Bit Score: 77.02 E-value: 1.85e-15
|
|||||||
| Dus | pfam01207 | Dihydrouridine synthase (Dus); Members of this family catalyze the reduction of the 5,6-double ... |
316-411 | 2.97e-10 | |||
Dihydrouridine synthase (Dus); Members of this family catalyze the reduction of the 5,6-double bond of a uridine residue on tRNA. Dihydrouridine modification of tRNA is widely observed in prokaryotes and eukaryotes, and also in some archae. Most dihydrouridines are found in the D loop of t-RNAs. The role of dihydrouridine in tRNA is currently unknown, but may increase conformational flexibility of the tRNA. It is likely that different family members have different substrate specificities, which may overlap. Dus 1 from Saccharomyces cerevisiae acts on pre-tRNA-Phe, while Dus 2 acts on pre-tRNA-Tyr and pre-tRNA-Leu. Dus 1 is active as a single subunit, requiring NADPH or NADH, and is stimulated by the presence of FAD. Some family members may be targeted to the mitochondria and even have a role in mitochondria. Pssm-ID: 426126 Cd Length: 309 Bit Score: 61.19 E-value: 2.97e-10
|
|||||||
| PRK10415 | PRK10415 | tRNA-dihydrouridine synthase B; Provisional |
310-416 | 6.82e-08 | |||
tRNA-dihydrouridine synthase B; Provisional Pssm-ID: 182440 Cd Length: 321 Bit Score: 53.82 E-value: 6.82e-08
|
|||||||
| Zn_finger_prot | pfam19242 | Putative zinc finger protein; This is a family of proteins predominantly found in Iridoviridae, ... |
55-161 | 7.56e-04 | |||
Putative zinc finger protein; This is a family of proteins predominantly found in Iridoviridae, which contains a zinc finger domain. Pssm-ID: 437074 Cd Length: 268 Bit Score: 41.21 E-value: 7.56e-04
|
|||||||
| Blast search parameters | ||||
|