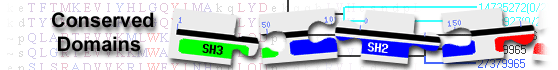|
Name |
Accession |
Description |
Interval |
E-value |
| PccA |
COG4770 |
Acetyl/propionyl-CoA carboxylase, alpha subunit [Lipid transport and metabolism]; |
63-358 |
0e+00 |
|
Acetyl/propionyl-CoA carboxylase, alpha subunit [Lipid transport and metabolism];
Pssm-ID: 443802 [Multi-domain] Cd Length: 466 Bit Score: 549.23 E-value: 0e+00
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 63 FDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTSKSYLNMDAIMEAIKKTRAQAVHPG 142
Cdd:COG4770 2 FKKVLIANRGEIAVRIIRTCRELGIRTVAVYSDADRDALHVRLADEAVCIGPAPAAESYLNIDAIIAAAKATGADAIHPG 81
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 143 YGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKASA 222
Cdd:COG4770 82 YGFLSENADFAEACEDAGIVFIGPSPEAIRAMGDKIAAKKLMKAAGVPVVPGSDGPVQDAEEALAIAEEIGYPVLIKASA 161
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 223 GGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQKVVEE 302
Cdd:COG4770 162 GGGGKGMRVVRSEEELEEAFESARREAKAAFGDDRVYLEKYIERPRHIEVQVLADKHGNVVHLGERDCSIQRRHQKVIEE 241
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*.
gi 2462537201 303 APSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNTRLQVLH 358
Cdd:COG4770 242 APSPALTEELRERMGEAAVRAAKAVGYVGAGTVEFLVDADGNFYFLEMNTRLQVEH 297
|
|
| PRK08591 |
PRK08591 |
acetyl-CoA carboxylase biotin carboxylase subunit; Validated |
62-358 |
3.26e-169 |
|
acetyl-CoA carboxylase biotin carboxylase subunit; Validated
Pssm-ID: 236307 [Multi-domain] Cd Length: 451 Bit Score: 479.30 E-value: 3.26e-169
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 62 TFDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTSKSYLNMDAIMEAIKKTRAQAVHP 141
Cdd:PRK08591 1 MFDKILIANRGEIALRIIRACKELGIKTVAVHSTADRDALHVQLADEAVCIGPAPSKKSYLNIPAIISAAEITGADAIHP 80
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 142 GYGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKAS 221
Cdd:PRK08591 81 GYGFLSENADFAEICEDSGFTFIGPSAETIRLMGDKVTAKATMKKAGVPVVPGSDGPVDDEEEALAIAKEIGYPVIIKAT 160
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 222 AGGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQKVVE 301
Cdd:PRK08591 161 AGGGGRGMRVVRTEAELEKAFSMARAEAKAAFGNPGVYMEKYLENPRHIEIQVLADGHGNAIHLGERDCSLQRRHQKVLE 240
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*..
gi 2462537201 302 EAPSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNTRLQVLH 358
Cdd:PRK08591 241 EAPSPAITEELRRKIGEAAVKAAKAIGYRGAGTIEFLYEKNGEFYFIEMNTRIQVEH 297
|
|
| PRK06111 |
PRK06111 |
acetyl-CoA carboxylase biotin carboxylase subunit; Validated |
63-358 |
1.42e-162 |
|
acetyl-CoA carboxylase biotin carboxylase subunit; Validated
Pssm-ID: 180406 [Multi-domain] Cd Length: 450 Bit Score: 462.58 E-value: 1.42e-162
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 63 FDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTSKSYLNMDAIMEAIKKTRAQAVHPG 142
Cdd:PRK06111 2 FQKVLIANRGEIAVRIIRTCQKLGIRTVAIYSEADRDALHVKMADEAYLIGGPRVQESYLNLEKIIEIAKKTGAEAIHPG 81
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 143 YGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKASA 222
Cdd:PRK06111 82 YGLLSENASFAERCKEEGIVFIGPSADIIAKMGSKIEARRAMQAAGVPVVPGITTNLEDAEEAIAIARQIGYPVMLKASA 161
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 223 GGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQKVVEE 302
Cdd:PRK06111 162 GGGGIGMQLVETEQELTKAFESNKKRAANFFGNGEMYIEKYIEDPRHIEIQLLADTHGNTVYLWERECSVQRRHQKVIEE 241
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*.
gi 2462537201 303 APSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNTRLQVLH 358
Cdd:PRK06111 242 APSPFLDEETRKAMGERAVQAAKAIGYTNAGTIEFLVDEQKNFYFLEMNTRLQVEH 297
|
|
| PRK08654 |
PRK08654 |
acetyl-CoA carboxylase biotin carboxylase subunit; |
63-360 |
2.08e-151 |
|
acetyl-CoA carboxylase biotin carboxylase subunit;
Pssm-ID: 236325 [Multi-domain] Cd Length: 499 Bit Score: 435.95 E-value: 2.08e-151
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 63 FDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTSKSYLNMDAIMEAIKKTRAQAVHPG 142
Cdd:PRK08654 2 FKKILIANRGEIAIRVMRACRELGIKTVAVYSEADKNALFVKYADEAYPIGPAPPSKSYLNIERIIDVAKKAGADAIHPG 81
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 143 YGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKASA 222
Cdd:PRK08654 82 YGFLAENPEFAKACEKAGIVFIGPSSDVIEAMGSKINAKKLMKKAGVPVLPGTEEGIEDIEEAKEIAEEIGYPVIIKASA 161
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 223 GGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQKVVEE 302
Cdd:PRK08654 162 GGGGIGMRVVYSEEELEDAIESTQSIAQSAFGDSTVFIEKYLEKPRHIEIQILADKHGNVIHLGDRECSIQRRHQKLIEE 241
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*...
gi 2462537201 303 APSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVdSKKNFYFLEMNTRLQVLHII 360
Cdd:PRK08654 242 APSPIMTPELRERMGEAAVKAAKAINYENAGTVEFLY-SNGNFYFLEMNTRLQVEHPI 298
|
|
| PRK05586 |
PRK05586 |
acetyl-CoA carboxylase biotin carboxylase subunit; |
63-360 |
1.31e-142 |
|
acetyl-CoA carboxylase biotin carboxylase subunit;
Pssm-ID: 180150 [Multi-domain] Cd Length: 447 Bit Score: 411.80 E-value: 1.31e-142
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 63 FDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTSKSYLNMDAIMEAIKKTRAQAVHPG 142
Cdd:PRK05586 2 FKKILIANRGEIAVRIIRACREMGIETVAVYSEADKDALHVQLADEAVCIGPASSKDSYLNIQNIISATVLTGAQAIHPG 81
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 143 YGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKASA 222
Cdd:PRK05586 82 FGFLSENSKFAKMCKECNIVFIGPDSETIELMGNKSNAREIMIKAGVPVVPGSEGEIENEEEALEIAKEIGYPVMVKASA 161
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 223 GGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQKVVEE 302
Cdd:PRK05586 162 GGGGRGIRIVRSEEELIKAFNTAKSEAKAAFGDDSMYIEKFIENPKHIEFQILGDNYGNVVHLGERDCSLQRRNQKVLEE 241
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*...
gi 2462537201 303 APSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNTRLQVLHII 360
Cdd:PRK05586 242 APSPVMTEELRKKMGEIAVKAAKAVNYKNAGTIEFLLDKDGNFYFMEMNTRIQVEHPI 299
|
|
| accC |
TIGR00514 |
acetyl-CoA carboxylase, biotin carboxylase subunit; This model represents the biotin ... |
63-360 |
1.04e-140 |
|
acetyl-CoA carboxylase, biotin carboxylase subunit; This model represents the biotin carboxylase subunit found usually as a component of acetyl-CoA carboxylase. Acetyl-CoA carboxylase is designated EC 6.4.1.2 and this component, biotin carboxylase, has its own designation, EC 6.3.4.14. Homologous domains are found in eukaryotic forms of acetyl-CoA carboxylase and in a number of other carboxylases (e.g. pyruvate carboxylase), but seed members and trusted cutoff are selected so as to exclude these. In some systems, the biotin carboxyl carrier protein and this protein (biotin carboxylase) may be shared by different carboxyltransferases. However, this model is not intended to identify the biotin carboxylase domain of propionyl-coA carboxylase. The model should hit the full length of proteins, except for chloroplast transit peptides in plants. If it hits a domain only of a longer protein, there may be a problem with the identification. [Fatty acid and phospholipid metabolism, Biosynthesis]
Pssm-ID: 129605 [Multi-domain] Cd Length: 449 Bit Score: 407.22 E-value: 1.04e-140
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 63 FDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTSKSYLNMDAIMEAIKKTRAQAVHPG 142
Cdd:TIGR00514 2 LDKILIANRGEIALRILRACKELGIKTVAVHSTADRDALHVLLADEAVCIGPAPSAKSYLNIPNIISAAEITGADAIHPG 81
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 143 YGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKASA 222
Cdd:TIGR00514 82 YGFLSENANFAEQCERSGFTFIGPSAESIRLMGDKVSAIETMKKAGVPCVPGSDGLVEDEEENVRIAKRIGYPVIIKATA 161
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 223 GGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQKVVEE 302
Cdd:TIGR00514 162 GGGGRGMRVVREPDELVKSISMTRAEAKAAFGNDGVYIEKYIENPRHVEIQVLADKYGNAIYLGERDCSIQRRHQKLLEE 241
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*...
gi 2462537201 303 APSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNTRLQVLHII 360
Cdd:TIGR00514 242 APSPALTPELRRKMGDAAVKAAVSIGYRGAGTVEFLLDKNGEFYFMEMNTRIQVEHPV 299
|
|
| PycA |
COG1038 |
Pyruvate carboxylase [Energy production and conversion]; Pyruvate carboxylase is part of the ... |
61-360 |
7.85e-137 |
|
Pyruvate carboxylase [Energy production and conversion]; Pyruvate carboxylase is part of the Pathway/BioSystem: Urea cycle
Pssm-ID: 440660 [Multi-domain] Cd Length: 1144 Bit Score: 417.56 E-value: 7.85e-137
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 61 KTFDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPA--PTsKSYLNMDAIMEAIKKTRAQA 138
Cdd:COG1038 2 KKIKKVLVANRGEIAIRVFRAATELGIRTVAIYSEEDRYSLHRFKADEAYLIGEGkgPV-DAYLDIEEIIRVAKEKGVDA 80
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 139 VHPGYGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMI 218
Cdd:COG1038 81 IHPGYGFLSENPEFARACEEAGITFIGPSPEVLEMLGDKVAARAAAIEAGVPVIPGTEGPVDDLEEALAFAEEIGYPVML 160
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 219 KASAGGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQK 298
Cdd:COG1038 161 KAAAGGGGRGMRVVRSEEELEEAFESARREAKAAFGDDEVFLEKYIERPKHIEVQILGDKHGNIVHLFERDCSVQRRHQK 240
|
250 260 270 280 290 300
....*....|....*....|....*....|....*....|....*....|....*....|..
gi 2462537201 299 VVEEAPSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNTRLQVLHII 360
Cdd:COG1038 241 VVEIAPAPNLDEELREAICEAAVKLAKAVGYVNAGTVEFLVDDDGNFYFIEVNPRIQVEHTV 302
|
|
| PRK12999 |
PRK12999 |
pyruvate carboxylase; Reviewed |
60-360 |
6.97e-134 |
|
pyruvate carboxylase; Reviewed
Pssm-ID: 237263 [Multi-domain] Cd Length: 1146 Bit Score: 409.91 E-value: 6.97e-134
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 60 EKTFDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVG--PAPtSKSYLNMDAIMEAIKKTRAQ 137
Cdd:PRK12999 2 MKKIKKVLVANRGEIAIRIFRAATELGIRTVAIYSEEDKLSLHRFKADEAYLIGegKHP-VRAYLDIDEIIRVAKQAGVD 80
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 138 AVHPGYGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVM 217
Cdd:PRK12999 81 AIHPGYGFLSENPEFARACAEAGITFIGPTAEVLRLLGDKVAARNAAIKAGVPVIPGSEGPIDDIEEALEFAEEIGYPIM 160
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 218 IKASAGGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQ 297
Cdd:PRK12999 161 LKASAGGGGRGMRIVRSEEELEEAFERAKREAKAAFGNDEVYLEKYVENPRHIEVQILGDKHGNVVHLYERDCSVQRRHQ 240
|
250 260 270 280 290 300
....*....|....*....|....*....|....*....|....*....|....*....|...
gi 2462537201 298 KVVEEAPSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNTRLQVLHII 360
Cdd:PRK12999 241 KVVEIAPAPGLSEELRERICEAAVKLARAVGYVNAGTVEFLVDADGNFYFIEVNPRIQVEHTV 303
|
|
| PRK08462 |
PRK08462 |
acetyl-CoA carboxylase biotin carboxylase subunit; |
65-360 |
1.74e-122 |
|
acetyl-CoA carboxylase biotin carboxylase subunit;
Pssm-ID: 236269 [Multi-domain] Cd Length: 445 Bit Score: 360.60 E-value: 1.74e-122
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 65 KILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTSKSYLNMDAIMEAIKKTRAQAVHPGYG 144
Cdd:PRK08462 6 RILIANRGEIALRAIRTIQEMGKEAIAIYSTADKDALYLKYADAKICIGGAKSSESYLNIPAIISAAEIFEADAIFPGYG 85
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 145 FLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKASAGG 224
Cdd:PRK08462 86 FLSENQNFVEICSHHNIKFIGPSVEVMALMSDKSKAKEVMKRAGVPVIPGSDGALKSYEEAKKIAKEIGYPVILKAAAGG 165
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 225 GGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQKVVEEAP 304
Cdd:PRK08462 166 GGRGMRVVEDESDLENLYLAAESEALSAFGDGTMYMEKFINNPRHIEVQILGDKHGNVIHVGERDCSLQRRHQKLIEESP 245
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*.
gi 2462537201 305 SIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNTRLQVLHII 360
Cdd:PRK08462 246 AVVLDEKTRERLHETAIKAAKAIGYEGAGTFEFLLDSNLDFYFMEMNTRLQVEHTV 301
|
|
| PRK12833 |
PRK12833 |
acetyl-CoA carboxylase biotin carboxylase subunit; Provisional |
61-358 |
4.91e-119 |
|
acetyl-CoA carboxylase biotin carboxylase subunit; Provisional
Pssm-ID: 183781 [Multi-domain] Cd Length: 467 Bit Score: 352.52 E-value: 4.91e-119
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 61 KTFDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTSKSYLNMDAIMEAIKKTRAQAVH 140
Cdd:PRK12833 3 SRIRKVLVANRGEIAVRIIRAARELGMRTVAACSDADRDSLAARMADEAVHIGPSHAAKSYLNPAAILAAARQCGADAIH 82
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 141 PGYGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKA 220
Cdd:PRK12833 83 PGYGFLSENAAFAEAVEAAGLIFVGPDAQTIRTMGDKARARRTARRAGVPTVPGSDGVVASLDAALEVAARIGYPLMIKA 162
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 221 SAGGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHgNALWLNERECSIQRRNQKVV 300
Cdd:PRK12833 163 AAGGGGRGIRVAHDAAQLAAELPLAQREAQAAFGDGGVYLERFIARARHIEVQILGDGE-RVVHLFERECSLQRRRQKIL 241
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*....
gi 2462537201 301 EEAPSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVD-SKKNFYFLEMNTRLQVLH 358
Cdd:PRK12833 242 EEAPSPSLTPAQRDALCASAVRLARQVGYRGAGTLEYLFDdARGEFYFIEMNTRIQVEH 300
|
|
| PRK07178 |
PRK07178 |
acetyl-CoA carboxylase biotin carboxylase subunit; |
63-360 |
6.16e-119 |
|
acetyl-CoA carboxylase biotin carboxylase subunit;
Pssm-ID: 180865 [Multi-domain] Cd Length: 472 Bit Score: 352.48 E-value: 6.16e-119
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 63 FDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTSkSYLNMDAIMEAIKKTRAQAVHPG 142
Cdd:PRK07178 2 IKKILIANRGEIAVRIVRACAEMGIRSVAIYSEADRHALHVKRADEAYSIGADPLA-GYLNPRRLVNLAVETGCDALHPG 80
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 143 YGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKASA 222
Cdd:PRK07178 81 YGFLSENAELAEICAERGIKFIGPSAEVIRRMGDKTEARRAMIKAGVPVTPGSEGNLADLDEALAEAERIGYPVMLKATS 160
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 223 GGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQKVVEE 302
Cdd:PRK07178 161 GGGGRGIRRCNSREELEQNFPRVISEATKAFGSAEVFLEKCIVNPKHIEVQILADSHGNVVHLFERDCSIQRRNQKLIEI 240
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*...
gi 2462537201 303 APSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNTRLQVLHII 360
Cdd:PRK07178 241 APSPQLTPEQRAYIGDLAVRAAKAVGYENAGTVEFLLDADGEVYFMEMNTRVQVEHTI 298
|
|
| PRK08463 |
PRK08463 |
acetyl-CoA carboxylase subunit A; Validated |
63-358 |
1.11e-106 |
|
acetyl-CoA carboxylase subunit A; Validated
Pssm-ID: 169452 [Multi-domain] Cd Length: 478 Bit Score: 321.38 E-value: 1.11e-106
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 63 FDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTsKSYLNMDAIMEAIKKTRAQAVHPG 142
Cdd:PRK08463 2 IHKILIANRGEIAVRVIRACRDLHIKSVAIYTEPDRECLHVKIADEAYRIGTDPI-KGYLDVKRIVEIAKACGADAIHPG 80
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 143 YGFLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRI-AREIGYPVMIKAS 221
Cdd:PRK08463 81 YGFLSENYEFAKAVEDAGIIFIGPKSEVIRKMGNKNIARYLMKKNGIPIVPGTEKLNSESMEEIKIfARKIGYPVILKAS 160
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 222 AGGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQKVVE 301
Cdd:PRK08463 161 GGGGGRGIRVVHKEEDLENAFESCKREALAYFNNDEVFMEKYVVNPRHIEFQILGDNYGNIIHLCERDCSIQRRHQKVIE 240
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*..
gi 2462537201 302 EAPSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNTRLQVLH 358
Cdd:PRK08463 241 IAPCPSISDNLRKTMGVTAVAAAKAVGYTNAGTIEFLLDDYNRFYFMEMNTRIQVEH 297
|
|
| CPSase_L_D2 |
pfam02786 |
Carbamoyl-phosphate synthase L chain, ATP binding domain; Carbamoyl-phosphate synthase ... |
176-358 |
3.85e-86 |
|
Carbamoyl-phosphate synthase L chain, ATP binding domain; Carbamoyl-phosphate synthase catalyzes the ATP-dependent synthesis of carbamyl-phosphate from glutamine or ammonia and bicarbonate. This important enzyme initiates both the urea cycle and the biosynthesis of arginine and/or pyrimidines. The carbamoyl-phosphate synthase (CPS) enzyme in prokaryotes is a heterodimer of a small and large chain. The small chain promotes the hydrolysis of glutamine to ammonia, which is used by the large chain to synthesize carbamoyl phosphate. See pfam00988. The small chain has a GATase domain in the carboxyl terminus. See pfam00117. The ATP binding domain (this one) has an ATP-grasp fold.
Pssm-ID: 397079 [Multi-domain] Cd Length: 209 Bit Score: 259.16 E-value: 3.85e-86
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 176 DKIESKLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKASAGGGGKGMRIAWDDEETRDGFRLSSQEAASSFGD 255
Cdd:pfam02786 1 DKVLFKAAMKEAGVPTVPGTAGPVETEEEALAAAKEIGYPVIIKAAFGGGGLGMGIARNEEELAELFALALAEAPAAFGN 80
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 256 DRLLIEKFIDNPRHIEIQVLGDKHGNALWLNERECSIQRRNQKVVEEAPSIFLDAETRRAMGEQAVALARAVKYSSAGTV 335
Cdd:pfam02786 81 PQVLVEKSLKGPKHIEYQVLRDAHGNCITVCNRECSDQRRTQKSIEVAPSQTLTDEERQMLREAAVKIARHLGYVGAGTV 160
|
170 180
....*....|....*....|....
gi 2462537201 336 EFLVDSK-KNFYFLEMNTRLQVLH 358
Cdd:pfam02786 161 EFALDPFsGEYYFIEMNTRLQVEH 184
|
|
| Biotin_carb_N |
pfam00289 |
Biotin carboxylase, N-terminal domain; This domain is structurally related to the PreATP-grasp ... |
63-170 |
2.94e-63 |
|
Biotin carboxylase, N-terminal domain; This domain is structurally related to the PreATP-grasp domain. The family contains the N-terminus of biotin carboxylase enzymes, and propionyl-CoA carboxylase A chain.
Pssm-ID: 425585 [Multi-domain] Cd Length: 108 Bit Score: 196.94 E-value: 2.94e-63
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 63 FDKILVANRGEIACRVIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVCVGPAPTSKSYLNMDAIMEAIKKTRAQAVHPG 142
Cdd:pfam00289 1 IKKVLIANRGEIAVRIIRACRELGIRTVAVYSEADANSLHVRLADEAVCLGPGPASESYLNIDAIIDAAKETGADAIHPG 80
|
90 100
....*....|....*....|....*...
gi 2462537201 143 YGFLSENKEFARCLAAEDVVFIGPDTHA 170
Cdd:pfam00289 81 YGFLSENAEFARACEEAGIIFIGPSPEA 108
|
|
| AccC |
COG0439 |
Biotin carboxylase [Lipid transport and metabolism]; Biotin carboxylase is part of the Pathway ... |
123-359 |
4.32e-49 |
|
Biotin carboxylase [Lipid transport and metabolism]; Biotin carboxylase is part of the Pathway/BioSystem: Fatty acid biosynthesis
Pssm-ID: 440208 [Multi-domain] Cd Length: 263 Bit Score: 165.82 E-value: 4.32e-49
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 123 NMDAIMEAIKKTRAQavHPGYGFLSENkEFARCLAAEdVV----FIGPDTHAIQAMGDKIESKLLAKKAEVNTiPGFDgV 198
Cdd:COG0439 1 DIDAIIAAAAELARE--TGIDAVLSES-EFAVETAAE-LAeelgLPGPSPEAIRAMRDKVLMREALAAAGVPV-PGFA-L 74
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 199 VKDAEEAVRIAREIGYPVMIKASAGGGGKGMRIAWDDEETRDGFRLSSQEAASSFGDDRLLIEKFIDNpRHIEIQVLGDk 278
Cdd:COG0439 75 VDSPEEALAFAEEIGYPVVVKPADGAGSRGVRVVRDEEELEAALAEARAEAKAGSPNGEVLVEEFLEG-REYSVEGLVR- 152
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 279 HGNALWlnereCSIQRRNQK---VVE---EAPSIfLDAETRRAMGEQAVALARAVKYS-SAGTVEFLVDSKKNFYFLEMN 351
Cdd:COG0439 153 DGEVVV-----CSITRKHQKppyFVElghEAPSP-LPEELRAEIGELVARALRALGYRrGAFHTEFLLTPDGEPYLIEIN 226
|
....*...
gi 2462537201 352 TRLQVLHI 359
Cdd:COG0439 227 ARLGGEHI 234
|
|
| CarB |
COG0458 |
Carbamoylphosphate synthase large subunit [Amino acid transport and metabolism, Nucleotide ... |
75-353 |
5.33e-18 |
|
Carbamoylphosphate synthase large subunit [Amino acid transport and metabolism, Nucleotide transport and metabolism]; Carbamoylphosphate synthase large subunit is part of the Pathway/BioSystem: Arginine biosynthesis
Pssm-ID: 440226 [Multi-domain] Cd Length: 536 Bit Score: 85.32 E-value: 5.33e-18
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 75 ACRVIRtckKMGIKTVAIHS-------DVDassvhvkMADeAVCVGPaptsksyLNMDAIMEAIKKTRAQAVHPGYG--- 144
Cdd:COG0458 21 ACKALR---EEGYEVILVNSnpetvstDYD-------TAD-RLYFEP-------LTVEDVLDIIEKEKPDGVIVQFGgqt 82
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 145 ------FLSENKEFarclaaEDVVFIGPDTHAIqamgDKIE-----SKLLaKKAEVNTIPGfdGVVKDAEEAVRIAREIG 213
Cdd:COG0458 83 alnlavELEEAGIL------EGVKILGTSPDAI----DLAEdrelfKELL-DKLGIPQPKS--GTATSVEEALAIAEEIG 149
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 214 YPVMIKASAGGGGKGMRIAWDDEE----TRDGFRLSsqeaassfGDDRLLIEKFIDNPRHIEIQVLGDKHGNALWLnere 289
Cdd:COG0458 150 YPVIVRPSYVLGGRGMGIVYNEEEleeyLERALKVS--------PDHPVLIDESLLGAKEIEVDVVRDGEDNVIIV---- 217
|
250 260 270 280 290 300 310
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*
gi 2462537201 290 CSIQrrNqkvVEEA-----------PSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKnFYFLEMNTR 353
Cdd:COG0458 218 GIME--H---IEPAgvhsgdsicvaPPQTLSDKEYQRLRDATLKIARALGVVGLCNIQFAVDDGR-VYVIEVNPR 286
|
|
| DdlA |
COG1181 |
D-alanine-D-alanine ligase or related ATP-grasp enzyme [Cell wall/membrane/envelope biogenesis, ... |
126-352 |
1.20e-13 |
|
D-alanine-D-alanine ligase or related ATP-grasp enzyme [Cell wall/membrane/envelope biogenesis, General function prediction only]; D-alanine-D-alanine ligase or related ATP-grasp enzyme is part of the Pathway/BioSystem: Mureine biosynthesis
Pssm-ID: 440794 [Multi-domain] Cd Length: 303 Bit Score: 70.52 E-value: 1.20e-13
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 126 AIMEAIKKTRAQAVhpgyGFLSENKEFARCLAAE--DVVFI--------------------------GPDTHAIqAMgDK 177
Cdd:COG1181 23 AVAAALDKAGYDVV----PIGIDVEDLPAALKELkpDVVFPalhgrggedgtiqgllellgipytgsGVLASAL-AM-DK 96
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 178 IESKLLAKKAEVNTIPGFdgVVKDAEEAV--RIAREIGYPVMIKASAGGGGKGMRIAWDDEETRDGFrlssqEAASSFgD 255
Cdd:COG1181 97 ALTKRVLAAAGLPTPPYV--VLRRGELADleAIEEELGLPLFVKPAREGSSVGVSKVKNAEELAAAL-----EEAFKY-D 168
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 256 DRLLIEKFIDnPRHIEIQVLGDKHGNALWLNErecsIQRRN-----------QKVVEEAPSIfLDAETRRAMGEQAVALA 324
Cdd:COG1181 169 DKVLVEEFID-GREVTVGVLGNGGPRALPPIE----IVPENgfydyeakytdGGTEYICPAR-LPEELEERIQELALKAF 242
|
250 260
....*....|....*....|....*...
gi 2462537201 325 RAVKYSSAGTVEFLVDSKKNFYFLEMNT 352
Cdd:COG1181 243 RALGCRGYARVDFRLDEDGEPYLLEVNT 270
|
|
| CPSaseII_lrg |
TIGR01369 |
carbamoyl-phosphate synthase, large subunit; Carbamoyl-phosphate synthase (CPSase) catalyzes ... |
78-353 |
2.10e-12 |
|
carbamoyl-phosphate synthase, large subunit; Carbamoyl-phosphate synthase (CPSase) catalyzes the first committed step in pyrimidine, arginine, and urea biosynthesis. In general, it is a glutamine-dependent enzyme, EC 6.3.5.5, termed CPSase II in eukaryotes. An exception is the mammalian mitochondrial urea-cycle form, CPSase I, in which the glutamine amidotransferase domain active site Cys on the small subunit has been lost, and the enzyme is ammonia-dependent. In both CPSase I and the closely related, glutamine-dependent CPSase III (allosterically activated by acetyl-glutamate) demonstrated in some other vertebrates, the small and large chain regions are fused in a single polypeptide chain. This model represents the large chain of glutamine-hydrolysing carbamoyl-phosphate synthases, or the corresponding regions of larger, multifunctional proteins, as found in all domains of life, and CPSase I forms are considered exceptions within the family. In several thermophilic species (Methanobacterium thermoautotrophicum, Methanococcus jannaschii, Aquifex aeolicus), the large subunit appears split, at different points, into two separate genes. [Purines, pyrimidines, nucleosides, and nucleotides, Pyrimidine ribonucleotide biosynthesis]
Pssm-ID: 273581 [Multi-domain] Cd Length: 1050 Bit Score: 68.49 E-value: 2.10e-12
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 78 VIRTCKKMGIKTVAIHSDVDASSVHVKMADeavcvgpaptsKSY---LNMDAIMEAIKKTRAQAVHPGYGFLSENKeFAR 154
Cdd:TIGR01369 580 AVLALRELGYETIMINYNPETVSTDYDTSD-----------RLYfepLTFEDVMNIIELEKPEGVIVQFGGQTPLN-LAK 647
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 155 CLAAEDVVFIGPDTHAIqamgDKIE-----SKLLaKKAEVNTIPGfdGVVKDAEEAVRIAREIGYPVMIKASAGGGGKGM 229
Cdd:TIGR01369 648 ALEEAGVPILGTSPESI----DRAEdrekfSELL-DELGIPQPKW--KTATSVEEAVEFASEIGYPVLVRPSYVLGGRAM 720
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 230 RIAWDDEETRDGFRlssqEAASSFGDDRLLIEKFIDNPRHIEIQVLGDkHGNALwlnerECSIQRRnqkvVEEA------ 303
Cdd:TIGR01369 721 EIVYNEEELRRYLE----EAVAVSPEHPVLIDKYLEDAVEVDVDAVSD-GEEVL-----IPGIMEH----IEEAgvhsgd 786
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|....*
gi 2462537201 304 -----PSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSkKNFYFLEMNTR 353
Cdd:TIGR01369 787 stcvlPPQTLSAEIVDRIKDIVRKIAKELNVKGLMNIQFAVKD-GEVYVIEVNPR 840
|
|
| ddl |
PRK01372 |
D-alanine--D-alanine ligase; Reviewed |
173-352 |
2.80e-12 |
|
D-alanine--D-alanine ligase; Reviewed
Pssm-ID: 234948 [Multi-domain] Cd Length: 304 Bit Score: 66.67 E-value: 2.80e-12
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 173 AMgDKIESKLLAKKAEVNTIPGfdGVVKDAEEAVRIAREIGYPVMIKASAGGGGKGMRIAWDDEETRDGFRLSSQEaass 252
Cdd:PRK01372 96 AM-DKLRTKLVWQAAGLPTPPW--IVLTREEDLLAAIDKLGLPLVVKPAREGSSVGVSKVKEEDELQAALELAFKY---- 168
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 253 fgDDRLLIEKFIDNPrhiEIQ--VLGDKhgnALwlnerecsiqrrnqKVVE-EAPSIF-------------------LDA 310
Cdd:PRK01372 169 --DDEVLVEKYIKGR---ELTvaVLGGK---AL--------------PVIEiVPAGEFydyeakylaggtqyicpagLPA 226
|
170 180 190 200
....*....|....*....|....*....|....*....|..
gi 2462537201 311 ETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNT 352
Cdd:PRK01372 227 EIEAELQELALKAYRALGCRGWGRVDFMLDEDGKPYLLEVNT 268
|
|
| COG3919 |
COG3919 |
Predicted ATP-dependent carboligase, ATP-grasp superfamily [General function prediction only]; |
78-354 |
2.94e-12 |
|
Predicted ATP-dependent carboligase, ATP-grasp superfamily [General function prediction only];
Pssm-ID: 443124 [Multi-domain] Cd Length: 382 Bit Score: 67.26 E-value: 2.94e-12
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 78 VIRTCKKMGIKTVAIHSDVDASSVHVKMADEAVcVGPAPTSKSYLNMDAIMEAIKKTRAQAVHPGY----GFLSENKEFA 153
Cdd:COG3919 20 VARSLGEAGVRVIVVDRDPLGPAARSRYVDEVV-VVPDPGDDPEAFVDALLELAERHGPDVLIPTGdeyvELLSRHRDEL 98
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 154 rclaAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFdgVVKDAEEAVRIAREIGYPVMIKASaggggkgMRIAW 233
Cdd:COG3919 99 ----EEHYRLPYPDADLLDRLLDKERFYELAEELGVPVPKTV--VLDSADDLDALAEDLGFPVVVKPA-------DSVGY 165
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 234 DD---EETRDGFRLSSQEA------ASSFGDDRLLIEKFIDNPRHIEIQVLG--DKHGNALWLnereCSIQRRNQKVVEE 302
Cdd:COG3919 166 DElsfPGKKKVFYVDDREEllallrRIAAAGYELIVQEYIPGDDGEMRGLTAyvDRDGEVVAT----FTGRKLRHYPPAG 241
|
250 260 270 280 290
....*....|....*....|....*....|....*....|....*....|...
gi 2462537201 303 APSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKN-FYFLEMNTRL 354
Cdd:COG3919 242 GNSAARESVDDPELEEAARRLLEALGYHGFANVEFKRDPRDGeYKLIEINPRF 294
|
|
| carB |
PRK05294 |
carbamoyl-phosphate synthase large subunit; |
153-274 |
1.72e-09 |
|
carbamoyl-phosphate synthase large subunit;
Pssm-ID: 235393 [Multi-domain] Cd Length: 1066 Bit Score: 59.34 E-value: 1.72e-09
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 153 ARCLAAEDVVFIGPDTHAIqamgDKIE-----SKLLaKKAEVNTIPGfdGVVKDAEEAVRIAREIGYPVMIKAS------ 221
Cdd:PRK05294 646 AKALEAAGVPILGTSPDAI----DLAEdrerfSKLL-EKLGIPQPPN--GTATSVEEALEVAEEIGYPVLVRPSyvlggr 718
|
90 100 110 120 130
....*....|....*....|....*....|....*....|....*....|...
gi 2462537201 222 AggggkgMRIAWDDEETRDGFRlssqEAASSFGDDRLLIEKFIDNPrhIEIQV 274
Cdd:PRK05294 719 A------MEIVYDEEELERYMR----EAVKVSPDHPVLIDKFLEGA--IEVDV 759
|
|
| CPSaseII_lrg |
TIGR01369 |
carbamoyl-phosphate synthase, large subunit; Carbamoyl-phosphate synthase (CPSase) catalyzes ... |
58-354 |
9.75e-09 |
|
carbamoyl-phosphate synthase, large subunit; Carbamoyl-phosphate synthase (CPSase) catalyzes the first committed step in pyrimidine, arginine, and urea biosynthesis. In general, it is a glutamine-dependent enzyme, EC 6.3.5.5, termed CPSase II in eukaryotes. An exception is the mammalian mitochondrial urea-cycle form, CPSase I, in which the glutamine amidotransferase domain active site Cys on the small subunit has been lost, and the enzyme is ammonia-dependent. In both CPSase I and the closely related, glutamine-dependent CPSase III (allosterically activated by acetyl-glutamate) demonstrated in some other vertebrates, the small and large chain regions are fused in a single polypeptide chain. This model represents the large chain of glutamine-hydrolysing carbamoyl-phosphate synthases, or the corresponding regions of larger, multifunctional proteins, as found in all domains of life, and CPSase I forms are considered exceptions within the family. In several thermophilic species (Methanobacterium thermoautotrophicum, Methanococcus jannaschii, Aquifex aeolicus), the large subunit appears split, at different points, into two separate genes. [Purines, pyrimidines, nucleosides, and nucleotides, Pyrimidine ribonucleotide biosynthesis]
Pssm-ID: 273581 [Multi-domain] Cd Length: 1050 Bit Score: 56.93 E-value: 9.75e-09
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 58 PNEKTFDKILVANRGEI----AC-------RVIRTCKKMGIKTVAIHSDVDASSVHVKMADEaVCVGPaptsksyLNMDA 126
Cdd:TIGR01369 1 PKRTDIKKILVIGSGPIvigqAAefdysgsQACKALKEEGYRVILVNSNPATIMTDPEMADK-VYIEP-------LTPEA 72
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 127 IMEAIKKTRAQAVHPGYG-----FLSENKEFARCLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGFdgVVKD 201
Cdd:TIGR01369 73 VEKIIEKERPDAILPTFGgqtalNLAVELEESGVLEKYGVEVLGTPVEAIKKAEDRELFREAMKEIGEPVPESE--IAHS 150
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 202 AEEAVRIAREIGYPVMIKASAGGGGKGMRIAWDDEE----TRDGFRLSSQeaassfgdDRLLIEKFIDNPRHIEIQVLGD 277
Cdd:TIGR01369 151 VEEALAAAKEIGYPVIVRPAFTLGGTGGGIAYNREElkeiAERALSASPI--------NQVLVEKSLAGWKEIEYEVMRD 222
|
250 260 270 280 290 300 310 320
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 278 KHGNALWLnereCSIQrrN----------QKVVeeAPSIFLDAETRRAMGEQAVALARAVKYSSAGTVEFLVDSK-KNFY 346
Cdd:TIGR01369 223 SNDNCITV----CNME--NfdpmgvhtgdSIVV--APSQTLTDKEYQMLRDASIKIIRELGIEGGCNVQFALNPDsGRYY 294
|
....*...
gi 2462537201 347 FLEMNTRL 354
Cdd:TIGR01369 295 VIEVNPRV 302
|
|
| carB |
PRK12815 |
carbamoyl phosphate synthase large subunit; Reviewed |
164-353 |
6.18e-08 |
|
carbamoyl phosphate synthase large subunit; Reviewed
Pssm-ID: 237215 [Multi-domain] Cd Length: 1068 Bit Score: 54.59 E-value: 6.18e-08
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 164 IGPDTHAIQAMGDKIESKLLAKKAEVNTIPGfdGVVKDAEEAVRIAREIGYPVMIKASAGGGGKGMRIAWDDEEtrdgfr 243
Cdd:PRK12815 658 LGTSPDTIDRLEDRDRFYQLLDELGLPHVPG--LTATDEEEAFAFAKRIGYPVLIRPSYVIGGQGMAVVYDEPA------ 729
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 244 LSSQEAASSFGDDRLLIEKFIDNpRHIEIQVLGDkhGNALWLN---ERecsiqrrnqkvVEEA-----------PSIFLD 309
Cdd:PRK12815 730 LEAYLAENASQLYPILIDQFIDG-KEYEVDAISD--GEDVTIPgiiEH-----------IEQAgvhsgdsiavlPPQSLS 795
|
170 180 190 200
....*....|....*....|....*....|....*....|....
gi 2462537201 310 AETRRAMGEQAVALARAVKYSSAGTVEFLVDSkKNFYFLEMNTR 353
Cdd:PRK12815 796 EEQQEKIRDYAIKIAKKLGFRGIMNIQFVLAN-DEIYVLEVNPR 838
|
|
| ddl |
PRK01966 |
D-alanine--D-alanine ligase; |
163-352 |
5.38e-07 |
|
D-alanine--D-alanine ligase;
Pssm-ID: 234993 [Multi-domain] Cd Length: 333 Bit Score: 50.89 E-value: 5.38e-07
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 163 FIGPDTHAiQAMG-DKIESKLLAKKAEVNTIPGfdgVV---KDAEEAV--RIAREIGYPVMIKASaggggkgmriawdde 236
Cdd:PRK01966 110 YVGCGVLA-SALSmDKILTKRLLAAAGIPVAPY---VVltrGDWEEASlaEIEAKLGLPVFVKPA--------------- 170
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 237 etRDGfrlssqeaaSSFG--------------------DDRLLIEKFIdNPRHIEIQVLGdkhgnalwlNERECSiqrrn 296
Cdd:PRK01966 171 --NLG---------SSVGiskvkneeelaaaldlafeyDRKVLVEQGI-KGREIECAVLG---------NDPKAS----- 224
|
170 180 190 200 210 220 230
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*..
gi 2462537201 297 qkVVEE--APSIFLD-------------------AETRRAMGEQAVALARAVKYSSAGTVEFLVDSKKNFYFLEMNT 352
Cdd:PRK01966 225 --VPGEivKPDDFYDyeakyldgsaeliipadlsEELTEKIRELAIKAFKALGCSGLARVDFFLTEDGEIYLNEINT 299
|
|
| PLN02735 |
PLN02735 |
carbamoyl-phosphate synthase |
166-281 |
7.50e-07 |
|
carbamoyl-phosphate synthase
Pssm-ID: 215391 [Multi-domain] Cd Length: 1102 Bit Score: 51.32 E-value: 7.50e-07
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 166 PDThaIQAMGDKIESKLLAKKAEVNTIPGfdGVVKDAEEAVRIAREIGYPVMIKASAGGGGKGMRIAWDDEETRdgfrlS 245
Cdd:PLN02735 694 PDS--IDAAEDRERFNAILNELKIEQPKG--GIARSEADALAIAKRIGYPVVVRPSYVLGGRAMEIVYSDDKLK-----T 764
|
90 100 110
....*....|....*....|....*....|....*..
gi 2462537201 246 SQEAASSFGDDR-LLIEKFIDNPRHIEIQVLGDKHGN 281
Cdd:PLN02735 765 YLETAVEVDPERpVLVDKYLSDATEIDVDALADSEGN 801
|
|
| PRK12767 |
PRK12767 |
carbamoyl phosphate synthase-like protein; Provisional |
105-353 |
2.43e-06 |
|
carbamoyl phosphate synthase-like protein; Provisional
Pssm-ID: 237195 [Multi-domain] Cd Length: 326 Bit Score: 48.73 E-value: 2.43e-06
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 105 MADEAVcVGPAPTSKSYLnmDAIMEAIKKTRAQAVHPGY----GFLSENKEFarcLAAEDVVFIGPDTHAIQAMGDKIES 180
Cdd:PRK12767 42 FADKFY-VVPKVTDPNYI--DRLLDICKKEKIDLLIPLIdpelPLLAQNRDR---FEEIGVKVLVSSKEVIEICNDKWLT 115
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 181 KLLAKKAEVNTIPGFDGVVKDAEEAVRIAREIGYPVMIKAsaggggkgmriawddeetRDG------FRLSSQEAASSFG 254
Cdd:PRK12767 116 YEFLKENGIPTPKSYLPESLEDFKAALAKGELQFPLFVKP------------------RDGsasigvFKVNDKEELEFLL 177
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 255 DDR--LLIEKFIDNPRhIEIQVLGDKHGNALwlnereCSIQRR---------NQKVVEEAPSIFldaetrramgEQAVAL 323
Cdd:PRK12767 178 EYVpnLIIQEFIEGQE-YTVDVLCDLNGEVI------SIVPRKrievragetSKGVTVKDPELF----------KLAERL 240
|
250 260 270
....*....|....*....|....*....|
gi 2462537201 324 ARAVKYSSAGTVEFLVDSKKnFYFLEMNTR 353
Cdd:PRK12767 241 AEALGARGPLNIQCFVTDGE-PYLFEINPR 269
|
|
| carB |
PRK12815 |
carbamoyl phosphate synthase large subunit; Reviewed |
156-354 |
2.52e-06 |
|
carbamoyl phosphate synthase large subunit; Reviewed
Pssm-ID: 237215 [Multi-domain] Cd Length: 1068 Bit Score: 49.58 E-value: 2.52e-06
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 156 LAAEDVVFIGPDTHAIQAMGDKIESKLLAKKaevNTIP-GFDGVVKDAEEAVRIAREIGYPVMIKASAGGGGKGMRIAWD 234
Cdd:PRK12815 108 LEQYGVELLGTNIEAIQKGEDRERFRALMKE---LGEPvPESEIVTSVEEALAFAEKIGFPIIVRPAYTLGGTGGGIAEN 184
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 235 DEETRDGFRlsSQEAASSFGDdrLLIEKFIDNPRHIEIQVLGDKHGNALWLNERE----CSIQRRNQKVVeeAPSIFL-D 309
Cdd:PRK12815 185 LEELEQLFK--QGLQASPIHQ--CLLEESIAGWKEIEYEVMRDRNGNCITVCNMEnidpVGIHTGDSIVV--APSQTLtD 258
|
170 180 190 200
....*....|....*....|....*....|....*....|....*.
gi 2462537201 310 AETRRaMGEQAVALARAVKYSSAGTVEFLVD-SKKNFYFLEMNTRL 354
Cdd:PRK12815 259 DEYQM-LRSASLKIISALGVVGGCNIQFALDpKSKQYYLIEVNPRV 303
|
|
| Dala_Dala_lig_C |
pfam07478 |
D-ala D-ala ligase C-terminus; This family represents the C-terminal, catalytic domain of the ... |
211-352 |
2.56e-06 |
|
D-ala D-ala ligase C-terminus; This family represents the C-terminal, catalytic domain of the D-alanine--D-alanine ligase enzyme EC:6.3.2.4. D-Alanine is one of the central molecules of the cross-linking step of peptidoglycan assembly. There are three enzymes involved in the D-alanine branch of peptidoglycan biosynthesis: the pyridoxal phosphate-dependent D-alanine racemase (Alr), the ATP-dependent D-alanine:D-alanine ligase (Ddl), and the ATP-dependent D-alanine:D-alanine-adding enzyme (MurF).
Pssm-ID: 429483 [Multi-domain] Cd Length: 204 Bit Score: 47.70 E-value: 2.56e-06
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 211 EIGYPVMIKASAGGGGKGMRIAWDDEETRDGFrlssqEAASSFgDDRLLIEKFIDNpRHIEIQVLGDKHGNALWLNER-- 288
Cdd:pfam07478 34 ALGYPVFVKPARLGSSVGVSKVESREELQAAI-----EEAFQY-DEKVLVEEGIEG-REIECAVLGNEDPEVSPVGEIvp 106
|
90 100 110 120 130 140
....*....|....*....|....*....|....*....|....*....|....*....|....*...
gi 2462537201 289 ECSIQRRNQKVVEEAPSIFLDAETRRAMGEQAVALA----RAVKYSSAGTVEFLVDSKKNFYFLEMNT 352
Cdd:pfam07478 107 SGGFYDYEAKYIDDSAQIVVPADLEEEQEEQIQELAlkayKALGCRGLARVDFFLTEDGEIVLNEVNT 174
|
|
| carB |
PRK05294 |
carbamoyl-phosphate synthase large subunit; |
75-282 |
2.01e-05 |
|
carbamoyl-phosphate synthase large subunit;
Pssm-ID: 235393 [Multi-domain] Cd Length: 1066 Bit Score: 46.63 E-value: 2.01e-05
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 75 ACRVIRtckKMGIKTVAIHSDV-----DAssvhvKMADeAVCVGPaptsksyLNMDAIMEAIKKTRAQAVHPGYG----- 144
Cdd:PRK05294 33 ACKALR---EEGYRVVLVNSNPatimtDP-----EMAD-ATYIEP-------ITPEFVEKIIEKERPDAILPTMGgqtal 96
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 145 ----FLSENKEFARClaaeDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPGfdGVVKDAEEAVRIAREIGYPVMIka 220
Cdd:PRK05294 97 nlavELAESGVLEKY----GVELIGAKLEAIDKAEDRELFKEAMKKIGLPVPRS--GIAHSMEEALEVAEEIGYPVII-- 168
|
170 180 190 200 210 220 230
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....
gi 2462537201 221 saggggkgmR-----------IAWDDEETRD----GFRLS--SQeaassfgddrLLIEKFIDNPRHIEIQVLGDKHGNA 282
Cdd:PRK05294 169 ---------RpsftlggtgggIAYNEEELEEiverGLDLSpvTE----------VLIEESLLGWKEYEYEVMRDKNDNC 228
|
|
| PurK |
COG0026 |
Phosphoribosylaminoimidazole carboxylase (NCAIR synthetase) [Nucleotide transport and ... |
83-265 |
1.10e-04 |
|
Phosphoribosylaminoimidazole carboxylase (NCAIR synthetase) [Nucleotide transport and metabolism]; Phosphoribosylaminoimidazole carboxylase (NCAIR synthetase) is part of the Pathway/BioSystem: Purine biosynthesis
Pssm-ID: 439797 [Multi-domain] Cd Length: 353 Bit Score: 43.52 E-value: 1.10e-04
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 83 KKMGIKTVAIHSDVDASSVHVkmADEAVcVGPaptsksYLNMDAIMEAIKktRAQAVhpgyGFLSEN--KEFARCLAAED 160
Cdd:COG0026 11 KRLGYRVHVLDPDPDSPAAQV--ADEHI-VAD------YDDEEALREFAE--RCDVV----TFEFENvpAEALEALEAEV 75
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 161 VVFigPDTHAIQAMGDKIESKLLAKKAEVNTiPGFDgVVKDAEEAVRIAREIGYPVMIKAsaggggkgmriawddeeTRD 240
Cdd:COG0026 76 PVR--PGPEALEIAQDRLLEKAFLAELGIPV-APFA-AVDSLEDLEAAIAELGLPAVLKT-----------------RRG 134
|
170 180 190
....*....|....*....|....*....|....*
gi 2462537201 241 G------FRLSSQE----AASSFGDDRLLIEKFID 265
Cdd:COG0026 135 GydgkgqVVIKSAAdleaAWAALGGGPCILEEFVP 169
|
|
| PRK06019 |
PRK06019 |
phosphoribosylaminoimidazole carboxylase ATPase subunit; Reviewed |
83-265 |
3.36e-04 |
|
phosphoribosylaminoimidazole carboxylase ATPase subunit; Reviewed
Pssm-ID: 235674 [Multi-domain] Cd Length: 372 Bit Score: 42.06 E-value: 3.36e-04
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 83 KKMGIKTVAIhsDVDASSVHVKMADEAVCVgpaptskSYLNMDAIMEAIKktRAQAVhpGYGFlsEN--KEFARCLAAED 160
Cdd:PRK06019 22 APLGYKVIVL--DPDPDSPAAQVADEVIVA-------DYDDVAALRELAE--QCDVI--TYEF--ENvpAEALDALAARV 86
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 161 VVFIGPDthAIQAMGDKIESKLLAKKAEVNTiPGFdGVVKDAEEAVRIAREIGYPVMIKasaggggkgmriawddeeTR- 239
Cdd:PRK06019 87 PVPPGPD--ALAIAQDRLTEKQFLDKLGIPV-APF-AVVDSAEDLEAALADLGLPAVLK------------------TRr 144
|
170 180 190
....*....|....*....|....*....|....*.
gi 2462537201 240 ---DG---FRLSS----QEAASSFGDDRLLIEKFID 265
Cdd:PRK06019 145 ggyDGkgqWVIRSaedlEAAWALLGSVPCILEEFVP 180
|
|
| PatZN |
COG1042 |
Acyl-CoA synthetase (NDP forming) [Energy production and conversion]; |
179-220 |
3.49e-04 |
|
Acyl-CoA synthetase (NDP forming) [Energy production and conversion];
Pssm-ID: 440664 [Multi-domain] Cd Length: 708 Bit Score: 42.42 E-value: 3.49e-04
10 20 30 40
....*....|....*....|....*....|....*....|..
gi 2462537201 179 ESKLLAKKAEVNTIPGfdGVVKDAEEAVRIAREIGYPVMIKA 220
Cdd:COG1042 492 EAKALLAAYGIPVVPT--RLARSAEEAVAAAEEIGYPVVLKI 531
|
|
| ATP-grasp_5 |
pfam13549 |
ATP-grasp domain; This family includes a diverse set of enzymes that possess ATP-dependent ... |
179-219 |
7.44e-04 |
|
ATP-grasp domain; This family includes a diverse set of enzymes that possess ATP-dependent carboxylate-amine ligase activity.
Pssm-ID: 463918 [Multi-domain] Cd Length: 221 Bit Score: 40.54 E-value: 7.44e-04
10 20 30 40
....*....|....*....|....*....|....*....|.
gi 2462537201 179 ESKLLAKKAEVNTIPGfdGVVKDAEEAVRIAREIGYPVMIK 219
Cdd:pfam13549 14 EAKALLAAYGIPVVPT--RLARSPEEAVAAAEEIGYPVVLK 52
|
|
| PRK14016 |
PRK14016 |
cyanophycin synthetase; Provisional |
197-264 |
1.23e-03 |
|
cyanophycin synthetase; Provisional
Pssm-ID: 237586 [Multi-domain] Cd Length: 727 Bit Score: 40.91 E-value: 1.23e-03
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 197 GVVKDAEEAVRIAREIGYPVMIKAsaggggkgmriawddeetRDG-------FRLSSQE-------AASSFGDDrLLIEK 262
Cdd:PRK14016 233 RVVTSAEDAWEAAEEIGYPVVVKP------------------LDGnhgrgvtVNITTREeieaayaVASKESSD-VIVER 293
|
..
gi 2462537201 263 FI 264
Cdd:PRK14016 294 YI 295
|
|
| PLN02735 |
PLN02735 |
carbamoyl-phosphate synthase |
129-354 |
3.78e-03 |
|
carbamoyl-phosphate synthase
Pssm-ID: 215391 [Multi-domain] Cd Length: 1102 Bit Score: 39.38 E-value: 3.78e-03
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 129 EAIKKTRAQAVHPGYG---FLSENKEFAR--CLAAEDVVFIGPDTHAIQAMGDKIESKLLAKKAEVNTIPgfDGVVKDAE 203
Cdd:PLN02735 92 QVIAKERPDALLPTMGgqtALNLAVALAEsgILEKYGVELIGAKLDAIKKAEDRELFKQAMEKIGLKTPP--SGIATTLD 169
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 204 EAVRIAREIG-YPVMIKASAGGGGKGMRIAWDDEETRDGFRLSSQEAASSfgddRLLIEKFIDNPRHIEIQVLGDKHGNA 282
Cdd:PLN02735 170 ECFEIAEDIGeFPLIIRPAFTLGGTGGGIAYNKEEFETICKAGLAASITS----QVLVEKSLLGWKEYELEVMRDLADNV 245
|
170 180 190 200 210 220 230 240
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 283 LWLnereCSIQRRNQKVVEEAPSI-------FLDAETRRaMGEQAVALARAVKYSSAGT-VEFLVDSKK-NFYFLEMNTR 353
Cdd:PLN02735 246 VII----CSIENIDPMGVHTGDSItvapaqtLTDKEYQR-LRDYSVAIIREIGVECGGSnVQFAVNPVDgEVMIIEMNPR 320
|
.
gi 2462537201 354 L 354
Cdd:PLN02735 321 V 321
|
|
| LysX |
COG0189 |
Glutathione synthase, LysX or RimK-type ligase, ATP-grasp superfamily [Amino acid transport ... |
152-351 |
4.25e-03 |
|
Glutathione synthase, LysX or RimK-type ligase, ATP-grasp superfamily [Amino acid transport and metabolism, Coenzyme transport and metabolism, Translation, ribosomal structure and biogenesis, Secondary metabolites biosynthesis, transport and catabolism]; Glutathione synthase, LysX or RimK-type ligase, ATP-grasp superfamily is part of the Pathway/BioSystem: Lysine biosynthesis
Pssm-ID: 439959 [Multi-domain] Cd Length: 289 Bit Score: 38.38 E-value: 4.25e-03
10 20 30 40 50 60 70 80
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 152 FARCLAAEDVVFIgPDTHAIQAMGDKIESKLLAKKAEVNTIPGFdgVVKDAEEAVRIAREIGYPVMIKasaggggkgmri 231
Cdd:COG0189 73 LLRQLEAAGVPVV-NDPEAIRRARDKLFTLQLLARAGIPVPPTL--VTRDPDDLRAFLEELGGPVVLK------------ 137
|
90 100 110 120 130 140 150 160
....*....|....*....|....*....|....*....|....*....|....*....|....*....|....*....|
gi 2462537201 232 awddeeTRDG------FRLSSQEAASSF-------GDDRLLIEKFIDNPRHIEIQ--VLGDKHGNALW--LNERECSIQR 294
Cdd:COG0189 138 ------PLDGsggrgvFLVEDEDALESIlealtelGSEPVLVQEFIPEEDGRDIRvlVVGGEPVAAIRriPAEGEFRTNL 211
|
170 180 190 200 210
....*....|....*....|....*....|....*....|....*....|....*..
gi 2462537201 295 RNQKVVEEAPsifLDAETRramgEQAVALARAVKYSSAGtVEFLVDsKKNFYFLEMN 351
Cdd:COG0189 212 ARGGRAEPVE---LTDEER----ELALRAAPALGLDFAG-VDLIED-DDGPLVLEVN 259
|
|
| |